This section is a transcript of the paper: Streaming waters: Challenges in monitoring the chemistry of dynamic environments. The authors analyze the challenges of chemical monitoring of Waters and call the attention of educators to the need to adapt the curricular contents for these issues. And our goal sharing this content is to show the need for automation of water quality monitoring.
What little survives from the writings of the philosopher Heraclitus (~535-475 B.C.E.) are collected as “Fragments” [1]. One of the most compelling can be translated to:
...one cannot step twice into the same river.
Whereas philosophically one might interpret this to mean that both the observer and the observed undergo constant change, in a scientific context, the meaning can be more literal. That is, nature is in a perpetual state of flux. Environmental waters are some of the more obvious examples of this-whether they be ocean, lake, river, or stream.
To understand their chemical composition requires measurements at frequencies that are high enough to create a realistic picture of the true state of the system.
As we describe in the following, this presents an opportunity to incorporate a novel type of instrument into the analytical chemistry curriculum. Students can learn to build simple monitors that illustrate the challenges (and frustrations) and benefits (and drawbacks) of remote environmental monitoring.
Although the concentrations of components in environmental waters change continuously over time, they must be sampled at discrete intervals. These discrete measurements are then used to reconstruct the original, continuous signal. Defining the appropriate sampling frequency can be difficult.
If one samples too frequently (over-samples), one can become burdened with unnecessary work and voluminous data. However, under-sampling can have even more dire consequences.
When discrete points that are taken too infrequently are then used to reconstruct by interpolation the continuous “true” signal, artifacts can be created. Creating artifacts as the result of the inaccurate interpolation is called “aliasing”. Figure 1 is an example of aliasing during a simulated monitoring period. The proper sampling frequency can be approached by applying the Nyquist-Shannon theorem, which is known as “the cardinal theorem of interpolation theory” [2]. The Nyquist-Shannon theorem requires one to sample at a rate that is at least twice the frequency of the true signal to prevent aliasing.
Figure 2. A simulated example of “under-sampling” in which weekly measurements (-•-) do not accurately reflect measurements made on a daily basis (-□-).
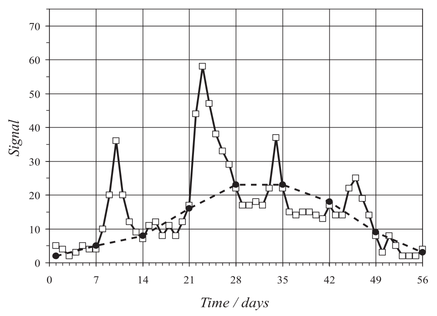
A more accurate understanding of a dynamic system will allow for a better understanding of the biogeochemical processes that occur therein. Given the dynamics of natural waters, however, and typical “grab” sampling methodologies, under-sampling is prone to occur in more rapidly changing environments. While concentrations of the chemical components that are found in the middle of a large lake or in a groundwater formation, for example, may vary little over relatively long periods of time, concentrations in tidal basins, rivers, and streams tend to fluctuate considerably.
To monitor dynamic environmental phenomena, ideally one would want to make the measurement directly at the point of sampling, or “in situ” because measurements can be made at high frequency. Not surprisingly, this has been an area of intensive research in analytical chemistry in recent years. [3] Early approaches, in which instruments designed for a laboratory environment were made more rugged for field use, failed because the instruments were expensive, bulky, fragile, etc.
More recent research has centered upon designing in situ instruments from the “bottom up”, and applying them to the determination of chemical species even at trace levels in complex matrices. [4]
Additionally, in situ measurements are immune from the possibility of sample contamination that occurs, at least to some degree, during transport to a laboratory and storage.
The ability of these instruments to self-calibrate is a crucial characteristic in maintaining high data quality.
While the quality of the data generated cannot match the precision and accuracy that is attainable with off-site fixed laboratory measurements, nor the power of sensitive multi-analyte determinations such as provided by GC-MS or ICP-MS, for many applications in situ monitoring instruments provide the desired quality of information.
Thus in situ instruments that have a capability of self-calibration can be particularly useful by attaining higher data quality.
In situ monitoring instrumentation can be divided into two general categories: “chemical sensors” and “chemical analyzers”. [5] Chemical sensors, in which mass transfer by diffusion brings the analyte to the transducer surface, are mechanically simplea nd practical in terms of their low power requirements, ruggedness, and small size and weight. Chemical sensors, however, often exhibit lower selectivity because their operation is based upon reversible chemical reactions. The interaction of the analyte with the sensor (transducer) surface must have a large binding (formation) constant K f if trace levels (sub-μg/mL) are to be measured. Consider the reaction of analyte A with ligand B to form complex AB:
[1] Kirk, G. S.; Raven, J. E.; Schofield, M. The Presocratic Philosophers, 2nd ed.; Cambridge University Press: Cambridge, U.K., 1983.
[2] Swanson, R.; Thoennes, D. J.; Williams, R. C.; Wilkins, C. L. Determination of the Nyquist Frequency. J. Chem. Educ. 1975, 52, 530.
[3] Valcarcel, M.; Cardenas, S.; Gallego, M. Continuous Flow Systems for Rapid Sample Screening. TrAC Trends Anal. Chem. 2002, 21, 251.
[4] Jannasch, H. W.; Johnson, K. S.; Sakamoto, C. M. Submersible, Osmotically Pumped Analyzers for Continuous Determination of Nitrate in situ. Anal. Chem. 1994, 66, 3352.
Bryne, R. H.; Kaltenbacher, E.; Waterbury, R. Autonomous in situ Analysis of the Upper Ocean. Sea Technol. 1999, 40, 71.
Lyddy-Meaney, A. J.; Ellis, P. S.; Worsfold, P. J.; Butler, E. C. V.; McKelvie, I. D. A Compact Flow Injection Analysis System for Surface Mapping of Phosphate in Marine Waters. Talanta 2002, 58, 1043.
Singer Pressman, M. A.; Aldstadt, J. H. An in situ Monitor Based on Continuous Flow Analysis for the Sensitive Quantitation of Hexavalent Chromium in Natural Waters. J. Environ. Monitoring 2005, 7, 809.
[5] Singer Pressman, M. A.; Aldstadt, J. H. In Situ Chemical Monitoring in Water Encyclopedia: Physics and Chemistry of Water (Vol.3); Lehr, J. H.; Keeley, J. (Eds.); John Wiley & Sons: New York, 2005.